 |
| Module 3: Toxicology - Section 1: Introduction to Occupational Toxicology |
| TOX 1.10: Toxicodynamics-: Classification of toxicity in laboratory experiments |
 |
| Module 3: Toxicology - Section 1: Introduction to Occupational Toxicology |
| TOX 1.10: Toxicodynamics-: Classification of toxicity in laboratory experiments |
In general, response is a function of the rate of elimination in relation to the rate of exposure.
Note also that the biological or clinical effect may be different at different exposure rates. In humans, this may result in the acute syndrome being very different from the chronic syndrome. One example is organophosphate poisoning in which the acute overexposure leads to the predicted effects of inhibition of acetylcholinesterase (to be covered in another session), while chronic lower dose exposures are associated with neurological disease and cancer.
Benzidine dyes are another example. Acute higher dose exposure results in methaemoglobinaemia, while the chronic lower dose exposure is associated with cancer of the bladder.
This is of relevance if you are reading the toxicological literature.
| Toxicity rating | Descriptive term | LD50 (wt/kg) single oral dose in rats | 4 hour inhalation LC50 in rats (ppm) |
|---|---|---|---|
| 1 | extremely toxic | £ 1mg | <10 |
| 2 | highly toxic | 1-50 mg | 10-100 |
| 3 | moderately toxic | 50 – 500 mg | 100 - 1000 |
| 4 | slightly toxic | 0.5 - 5 g | 1000 - 10000 |
| 5 | practically nontoxic | 5 - 15 g | 10000 - 100000 |
| 6 | relatively harmless | ³ 15g | >100000 |
Note that in the animal model, route of administration, dose and time pattern needs to be specified, e.g. 1000 ppm over 7 hrs, or 3800 mg/kg. The result may then be expressed as "oral-rat LD50 = 3800 mg/kg (3000, 4600)", or "inhale-rat LC50 =1000 ppm / 7 hrs (750, 1250)".
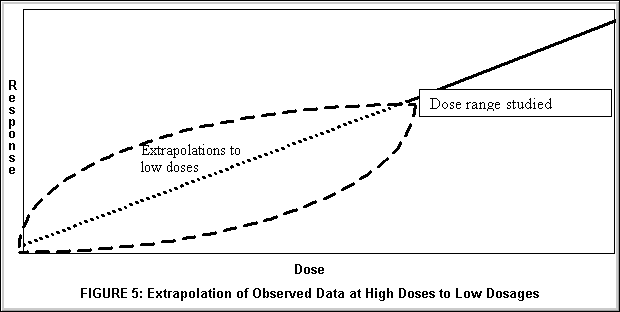
Figure 5 above illustrates another method for interpreting laboratory toxicology studies. Given the large number of subjects needed to observe an unlikely phenomenon, one usually cannot afford to study sufficient numbers of subjects at very low doses to accurately portray the dose response relationship at the lowest dose ranges. Hence laboratory animals are typically studied at dosages which are orders of magnitude higher than those to which humans are expected to be exposed. Conclusions of risk in human settings are then derived from extrapolations of observed data at higher ranges to the lower ranges at which humans might be exposed.

Postgraduate Diploma in Occupational Health (DOH) - Modules 3: Occupational Medicine & Toxicology (Basic) by Profs Mohamed Jeebhay and Rodney Ehrlich, Health Sciences UCT is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.5 South Africa License. Major contributors: Mohamed Jeebhay, Rodney Ehrlich, Jonny Myers, Leslie London, Sophie Kisting, Rajen Naidoo, Saloshni Naidoo. Source available from here. For any updates to the material, or more permissions beyond the scope of this license, please email healthoer@uct.ac.za or visit www.healthedu.uct.ac.za.
Last updated Jan 2007.
Disclaimer note: Some resources and descriptions may be out-dated. For suggested updates and feedback, please contact healthoer@uct.ac.za.