
 |
| Block 8: Environmental Issues and Public Health - Air Pollution Chapter 1: Introduction (Continued) |
The now famous Fog of London of 1952 demonstrated the health impact (about 4 000 excess deaths occurred during the 5-day period on the Fog) of these severe air pollution episodes, yielded considerable data (Figure 4) and spurred the development of more effective legislation to control air pollution.
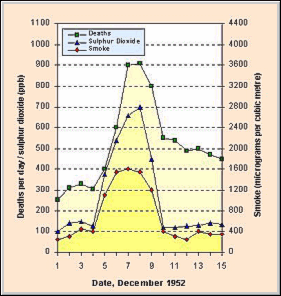 |
Figure 4 sµggests an association between the increase in sulphur dioxide and smoke concentrations and mortality rates. The concentrations increased from about 130 µg.m-3 (SO2) and 400 µg.m-3 (smoke) during 1 to 4 December 1952, to peaks of 700 µg.m-3 (SO2) and 1600 µg.m-3 (smoke) on 7-8th December 1952.
During 7-8th December, the death rate peaked at about 900 per day compared with about 300 per day prior to the pollution episode. The deaths were ascribed to pneumonia, asthma and bronchitis. |
| Figure 4: Death rates and pollutant concentrations during the "Fog of London" (1952) |
| Over the last 50 years or so, a large number of studies demonstrated the cause-effect relationship between specific air pollutants and specific health endpoints; in many cases exposure-response relationships have been derived as well. The early studies focussed on the acute health effects (those occurring within days of exposure) of short-term exposure (exposure durations ranging from a few minutes to several days) to the ‘classical’ pollutants, namely sulphur dioxide (SO2), particulate matter (PM), oxides of nitrogen (NOx), ozone (O3), carbon monoxide (CO) and lead (Pb). In the USA, these six pollutants are called "criteria" pollutants. These six pollutants, and volatile organic compounds (VOCs), are the main pollutants arising out of the combustion of fossil fuels (coal and oil products), and are generated in industrial power plants, petrol and diesel driven vehicles and by domestic cooking and heating appliances. The relative contribution (to ambient concentrations) of each of these three broad air pollution source categories varies from city to city.
An overall assessment of ambient pollutant concentrations (and their impacts) must include a consideration of secondary air pollutants - air pollutants that are not directly emitted but are formed throµgh atmospheric chemical and physical processes. Ozone (in the troposphere, close to the earth’s surface) is formed throµgh chemical reactions involving VOCs and NOx, under the influence of sunlight. Fine (PM2.5, particulate matter less than 2.5 um diameter) particulate matter, mainly sulphates and nitrates, are formed due to the presence of SO2 and NOx as precursors. |
VOCs: hydrocarbon compounds NOx: nitrogen dioxide (NO2) and nitric oxide (NO).NOx is formed during combustion processes; about 90% of the NOx is release as NO which is converted to NO2 in the atmosphere. Sulphates: sulphuric acid (H2SO4) droplets, ammonium bisulphate, etc. Nitrates: The reaction of NO2 and O3 may produce nitric acid (HNO3) droplets and/ or peroxyacetyl nitrate (PAN) |
The health impacts associated with ozone and PM2.5 are significant, and may have a greater contribution to specific health endpoints than that of the primary air pollutants.
In addition to the acute health impacts due to short-term exposure to air pollutants the health risks associated with prolonged exposure, including cancer and developmental risks, have to be considered as well. In the case of certain air pollutants, such as heavy metals (compounds of lead, chromium, etc.) and dioxins/ furans, the primary exposure path for people is not the direct inhalation of the polluted air, but through contaminated food or dust.
| These persistent air pollutants (they do not break down into less toxic substances naturally, or break down very slowly) settle on crops or grass that is in turn eaten by livestock and subsequently by people. Water and sediments contaminated by persistent toxic substances result in the contamination of aquatic species and the food web, with attendant environmental and health risk consequences. Children may ingest contaminated dust - this is the main exposure pathway for leaded petrol emissions. A full assessment of the health risks associated with these substances requires a consideration of all pathways of exposure. | POPs: Persistent Organic Pollutants PTS: Persistent Toxic Substances - includes POPs and metal compounds |
In the absence of direct measures of the dose of the pollutants received (for example through measurement of biomarkers such as lead-in-blood levels), exposure-response relationships (frequently called dose-response relationships) may be used to estimate potential impacts on people and/or the environment. The World Health Organisation (WHO) reviewed summarised and published information on the exposure-response relationships for the most commonly encountered urban air pollutants as well as Air Quality Guidelines Values.
The accidental release of substantial quantities of air pollutants from industrial plants may occur relatively infrequently, but may have a significant impact on the surrounding communities. These industrial accidental may result in fires, explosions and /or the release of toxic substances, contributing to overall exposure and impact of these operations. The assessment of this type of health and safety risk requires additional risk assessment methodologies, including estimates of the probability of the occurrence of the accident, and the consequences (impacts), of a given accident scenario.
| Both industrialised and semi-industrialised cities are subjected to a wide variety of sources of air pollution, and many more air pollutants - more than to 200. A number of countries compile and publish inventories of (annual) air pollutant emissions. The US EPA monitors and reports on six "Criteria" pollutants. | US EPA: United States’ Environmental Protection Agency |
These criteria polutants are: sulphur dioxide (SO), particulate matter (PM), oxides of nitrogen (NOx), ozone (O3), carbon monoxide (CO) and lead (Pb)) and 189 Hazardous Air Pollutants. The latter list includes dioxins, furans and compounds of mercury, lead, chromium and a number of organic compounds. Canada, Mexico and a number of other countries compile similar air pollutant inventories. Industrial processes such as oil refining and waste incineration generate their own profile of pollutants. The complexity arising out of multiple sources of air pollution and the large number of pollutants that may be discharged from different sources means that it is usually difficult to unequivocally apportion ambient concentrations (and hence health impacts) at a particular location to specific sources.
Sources of air pollution may be characterized by factors such as the emission rates of specific pollutants, whether the source is stationary or mobile, the elevation of the source in relation to environmental receptors (people, crops, buildings etc.), and the gas exit velocity and temperature if the source is emitted from a stack. Stationary sources may be further characterized as point sources - chimneys or stacks - and area sources such as a landfill sites, or agricultural areas. Emissions from mobile sources, essentially emissions from petrol or diesel fuelled vehicles, depend on vehicle type and size, travelling speed, the fuel quality and total kilometres travelled. Different measurement and estimation techniques may be used to estimate pollutant emission rates from the different source types. Several factors not directly related to pollution source characteristics influence the ‘instantaneous’ (in practice, measured over a short but finite time period of a few seconds to a few minutes) ambient concentration of air pollutants and the concentrations averaged over a given time period (the averaging times are usually 15 minutes up to a year) at a give location. These factors are mainly the meteorological conditions, distance from the source and the nature of the intervening terrain - whether urban, rural or a water body. The health and environmental impact of an air pollution source depends on several factors. |
ppm: parts per million, by volume. That is, the ratio of the volume of gaseous pollutant to the volume of air in a mixture. ppb: ppm/1000 Units of ppm and ppb are commonly used as expressions of concentration but they are dimensionless ratios. Particulate Matter (PM) concentrations are always expressed in mass/volume terms. |
Human exposure may be defined as the contact of the (air) pollutant with a susceptible surface of the body, including the eyes, nose, mouth and throat, the skin, the airways and the lungs. Air pollution exposure is characterised by the concentration of the pollutant and the averaging period over which the concentration has been measured. Averaging periods in use are ‘instantaneous’ (in practice, a few seconds to a few minutes), 10 or 15 minutes, 1, 3 or 8 hours, 24 hours or 1 year. Seasonal (summer/ winter) averages are sometimes relevant. The pollutant concentration may be expressed as mass per unit volume of air (µg or mg/m3), or as a mixing ratio or volume ratio (ppm or ppb). An continuous concentration-time function provides a more complete measure (metric) of exposure; the cumulative exposure (the sum or integral of the concentration-time function over a given time period) may be the relevant measure of exposure for cumulative toxic substances such as lead.
The exposure of crops, vegetation, livestock and physical infrastructure (buildings, bridges, electricity pylons, etc.) to pollutants may result in economic losses due to a decrease in crop yields, corrosion of steel structures and the deterioration of paintwork; exposure of vegetation or the acidification of rivers, water bodies (lakes) and soil may lead to damage to natural fauna and flora; contamination of grazing and livestock fodder may lead to further human exposure to certain pollutants.
A complex set of factors relates the sources of air pollution to human exposure and to environmental degradation, including the atmospheric processes of dispersion, chemical and physical transformation, and deposition of pollutants onto surfaces. A basic understanding of these factors is necessary for the management and protection of the (urban) environment.
An effective management system requires a comprehensive Air Quality Management System and an effective legislative and regulatory environment. International treaties (Conventions) are becoming increasingly important to the safe management of hardous chemicals and hazardous wastes.