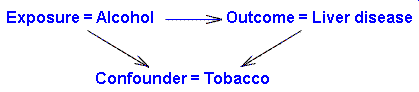|
| EPI10-1: Confounding |
OBJECTIVES |
At the end of this session you should:
- understand the definition of confounding;
- be able to examine a table of data providing the full data for an exposure of interest and a potential confounder in subjects with and without the disease and to perform stratified analysis of this data to examine for confounding;
- understand the definition of effect modification;
- be able to examine a table of data providing the full data for an exposure of interest and a potential effect modifier (EM) in subjects with and without the disease and to perform stratified analysis of this data to examine for effect modification;
- understand the difference between confounding and effect modification.
|
CONFOUNDING:
Confounding refers to the effect of an extraneous variable on the association between exposure and the outcome of interest.
Example:
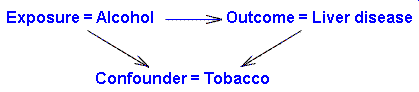
More generally for something to be a confounder three conditions must be satisfied:
- The confounder must be associated with the outcome in the unexposed.
- The confounder must be associated with the exposure.
- The confounder must not be an intermediate variable in a causal chain from exposure to outcome.
Confounding can be controlled either by study design eg. a randomised controlled trial, or in the analysis after the study has been conducted eg by stratified analysis or by standardisation or multivariate analysis.