 |
| Module 3: Toxicology - Section 1: Introduction to Occupational Toxicology |
| TOX 1.7: Toxicokinetics - Absorption |
 |
| Module 3: Toxicology - Section 1: Introduction to Occupational Toxicology |
| TOX 1.7: Toxicokinetics - Absorption |
The concentration of a toxin and its effects depend on the following:
Both the concentration and lipid solubility of the toxic substance determine the rate of absorption. Increased blood flow and large surface areas of exposure increase the rate of absorption. Common routes of absorption are the gastrointestinal tract, respiratory tract and skin or dermal system.
Toxic substances that are ingested are absorbed mostly in the small intestine. However, relatively acidic substances such as aspirin are in an ionized state in alkaline environments and non-ionized in acidic environments. Hence aspirin is well absorbed in the stomach, an acidic environment.
The most common route of exposure in occupational settings is the respiratory tract. The degree of water solubility and ionization of the toxic substance determines its point of absorption in the respiratory tract.
Skin absorption is dependent on the degree of contact of the toxic agent and its lipid solubility. Aspects such as hydration of the dermis, open lesions and thickness of the skin affect the extent of absorption. The greatest rate of dermal absorption is in the axilla and perineum and in areas of dermatitis.
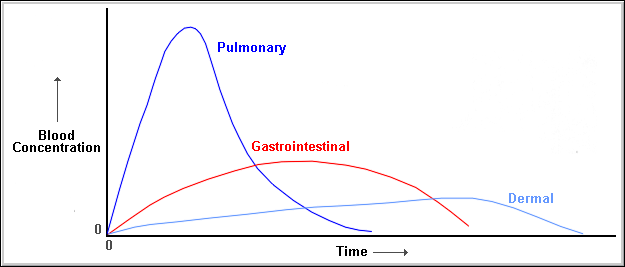
The above diagram reflects the route of maximum absorption over time showing that the most effective route of absorption is via the respiratory route, ( for particles small enough to enter the lower respiratory tract), followed by the gastrointestinal tract, with the least effective route being through the skin.
The skin is both a "barrier" and a significant route of absorption. Passive diffusion is the major mechanism of absorption and correlates with the partition coefficient or lipid solubility of an agent. Abrasion of the skin and dermatitis increase permeability.
In general, the stratum corneum is relatively impermeable (palms and soles have thick but fractured and very permeable stratum corneum). The scrotum has very thin and permeable stratum corneum. Organic solvents pass readily across the skin and may act as vehicles for absorption of pesticides.
Skin permeability is complex and difficult to predict. The most skin permeable substances are those that are neither too fat soluble nor too water soluble, in other words, somewhere in between.
The lungs have a surface area of 50 to 100 m2 with great proximity of the alveolar capillary vasculature to the alveolar air space maximizing gas exchange. Water solubility and particle size determine the depth of penetration of a substance.
Water soluble substances largely go into solution in the mucous lining of the upper respiratory tract. The particle size (strictly, the aerodynamic diameter) of the chemical determines site of deposition in the airway:
Absorption of a substance to particulate such as sulphur dioxide adhering to coal soot particulate, may enhance both depth of penetration and residence time in the lung.
A great many chemicals are absorbed when ingested. Gastrointestinal absorption may be deliberate as in the case of poisoning, or secondary due to hand mouth contact or following mucociliary clearance from the upper respiratory tract.
The gut has a large surface area and relatively high rate of blood supply. This means that absorbed substances are quickly moved and a concentration gradient between the gut lumen and the blood is maintained to ensure high levels of absorption. Diet and transit time can both affect the rate or effectiveness of absorption.
Bacteria in the gut can also biotransform and increase the toxicity of certain chemicals. E.g. nitrates are reduced to nitrites by E. coli (R-O-NO2 to R-O-NO), which may result in methaemoglobinemia (and may react with secondary amines to form nitrosamines, which may be carcinogenic). This occurs primarily in infants with low acidity in their stomachs and the elderly with achlorhydria (lack ofacid).
Uncommon, but may occur via splashes, e.g. organophosphate insecticides.

Postgraduate Diploma in Occupational Health (DOH) - Modules 3: Occupational Medicine & Toxicology (Basic) by Profs Mohamed Jeebhay and Rodney Ehrlich, Health Sciences UCT is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.5 South Africa License. Major contributors: Mohamed Jeebhay, Rodney Ehrlich, Jonny Myers, Leslie London, Sophie Kisting, Rajen Naidoo, Saloshni Naidoo. Source available from here. For any updates to the material, or more permissions beyond the scope of this license, please email healthoer@uct.ac.za or visit www.healthedu.uct.ac.za.
Last updated Jan 2007.
Disclaimer note: Some resources and descriptions may be out-dated. For suggested updates and feedback, please contact healthoer@uct.ac.za.