 |
| Module 3: Toxicology - Section 2: General Principles of Toxicology |
| TOX2.3: Toxicokinetics |
 |
| Module 3: Toxicology - Section 2: General Principles of Toxicology |
| TOX2.3: Toxicokinetics |
The concentration of a toxin and its effects depend on the following:
Each have specialized active transport mechanism for nutrient supply and for excretion of organic acids and bases.
The passage of chemicals through the body is a dynamic process and is can be described using compartmental models.
The chemical is distributed through out the body and the concentration in plasma represents the concentration in the compartment.
The central compartment represents the vascular space and the rapidly perfused tissues while the peripheral compartment represents the rest of the body.
We denote Kab as the constant rate of absorption of the chemical, and,The volume in which a chemical is dispersed depends on its solubility in water and if it binds to plasma proteins or intracellular sites.
The volume of distribution (Vd) relates the amount of chemical in the body to the concentration of chemical in the blood. Chemicals bound to plasma proteins are in the plasma and thus the Vd is small.
The body burden of a chemical = plasma concentration of a chemical x Vd
The clearance, (Clt) is a measure of the overall efficiency of the removal of a chemical from the body.
Clt = Vd x Kel
If chemical elimination occurs by a first order process in which the chemical removed per unit time remains constant it will occur exponentially. The plasma half life is inversely proportional to the Kel.
T½ = 0.693/Kel
If elimination is through a saturable process then the rate of elimination follows zero-order kinetics (a constant rate is eliminated).
With repeated exposure, assuming first order elimination, the amount of chemical eliminated will equal the amount absorbed. As a result, a steady state will be reached, and is reflected by the formula:
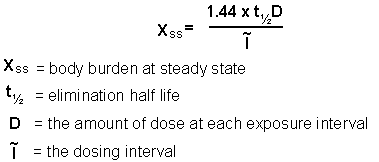

Postgraduate Diploma in Occupational Health (DOH) - Modules 3: Occupational Medicine & Toxicology (Basic) by Profs Mohamed Jeebhay and Rodney Ehrlich, Health Sciences UCT is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.5 South Africa License. Major contributors: Mohamed Jeebhay, Rodney Ehrlich, Jonny Myers, Leslie London, Sophie Kisting, Rajen Naidoo, Saloshni Naidoo. Source available from here. For any updates to the material, or more permissions beyond the scope of this license, please email healthoer@uct.ac.za or visit www.healthedu.uct.ac.za.
Last updated Jan 2007.
Disclaimer note: Some resources and descriptions may be out-dated. For suggested updates and feedback, please contact healthoer@uct.ac.za.